According to the Supervision and Administration Regulations (CSAR), the efficacy claims of cosmetics should have a sufficient scientific basis. Cosmetics registrant and filer should publish a summary of the literature, research data or product efficacy evaluation information on the efficacy claims to the NMPA designated website for public supervision.
Cosmetic registrant and filer should be responsible for the scientificity, authenticity, reliability and traceability of the submitted summary of the basis for the efficacy claims.
Exemption of Efficacy Evaluation
Sensory recognition by the way of visual or olfactory system
-ex. cleaning, makeup removal, beauty and modification, fragrance, refreshing, hair dye, hair perm, hair color care, depilation, deodorization, auxiliary shaving. etc.
Physical effect by the way of covering, adhesion, friction
-ex. whitening, exfoliator, deep pore cleanser (labelled as having physical effect only)
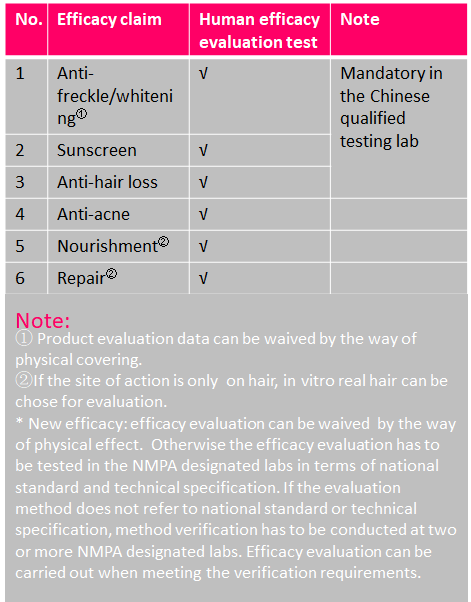
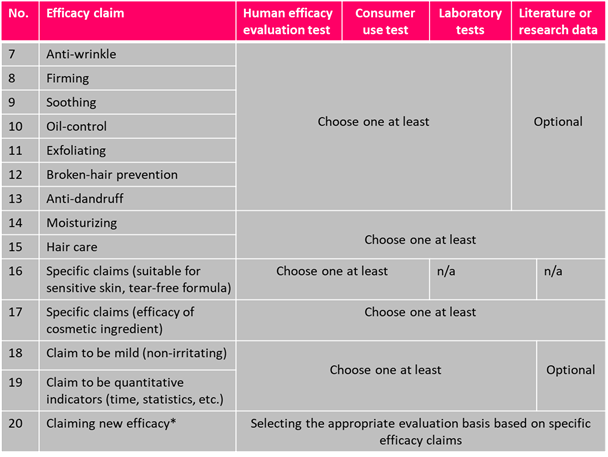
Efficacy Evaluation Requirements of Cosmetics