A breakthrough study on the anti-photodamage effects of Gardenia Fructus Extracts, jointly conducted by Hangzhou CIRS KEXIN Biotech Co., Ltd. (CIRS Biotech) and Shanghai Junyu Biotechnology Co., Ltd. (Junyu Group), has been published in Cosmetics, an internationally renowned SCI-indexed journal. The breakthrough study provides scientific validation for the development of photoprotective ingredients and the establishment of its efficacy evaluation systems.
Utilizing a multidisciplinary methodology integrating in vitro efficacy assessment, proteomics, and network pharmacology, this research elucidates the anti-photodamage mechanisms of Gardenia Fructus Extract. The extract demonstrably mitigates UVB-induced photodamage through multipathway regulation: enhancing antioxidant defenses, suppressing inflammatory cascades, accelerating barrier restoration, and modulating cell cycle progression (specifically via G2/M checkpoint control and p53 signaling). Geniposin was established as the primary bioactive constituent. This work provides an evidence-based paradigm for screening natural photoprotective cosmetic ingredients.
As a professional third-party research institution, CIRS Biotech devotes itself to providing scientific validation for cosmetic efficacy claims to cosmetics and ingredients companies. It has established comprehensive technical systems across core efficacy evaluation domains—including whitening, anti-aging, and sensitive skin solutions—delivering one-stop solutions, from ingredient screening and in vitro efficacy evaluation to product development testing and technical consulting. Junyu Group, a leader in beauty and health sciences, leverages core expertise in plant extract bioactivity and Traditional Chinese Medicine (TCM) modernization. Based on mutual research objectives, the two companies carried out the research on the photoprotective effective and potential mechanisms of gardenia fructus extract in UVB-irradiated HaCaT cells, with the aim to bridge traditional medicine with modern skincare innovation.

Key Research Innovations
1. Multi-dimensional Evaluation System
An integrated assessment system was established by combining the antioxidant enzyme activities (T-SOD/CAT/GSH-Px), lipid peroxidation marker (MDA) quantification, and 3D epidermal model barrier protein (FLG/LOR/IVL) immunofluorescence imaging. This comprehensive system enables quantitative evaluation of oxidative stress → inflammatory response → barrier repair dynamics across cellular and tissue hierarchies.
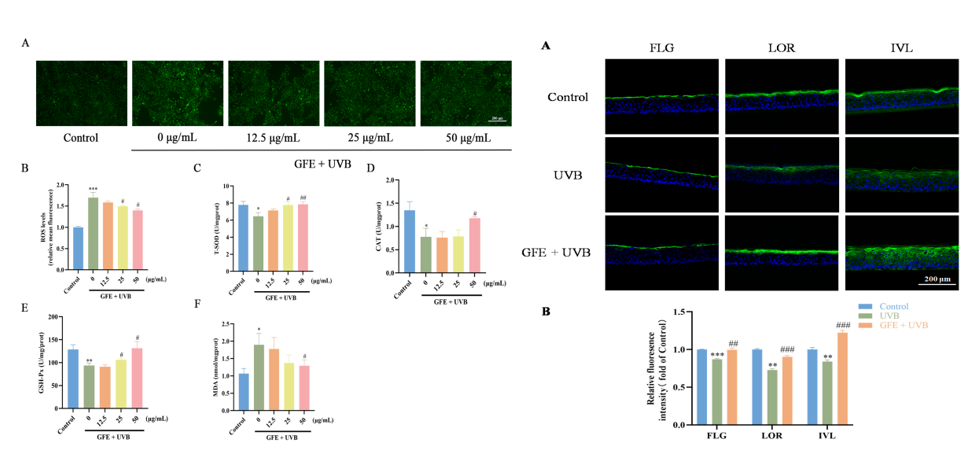
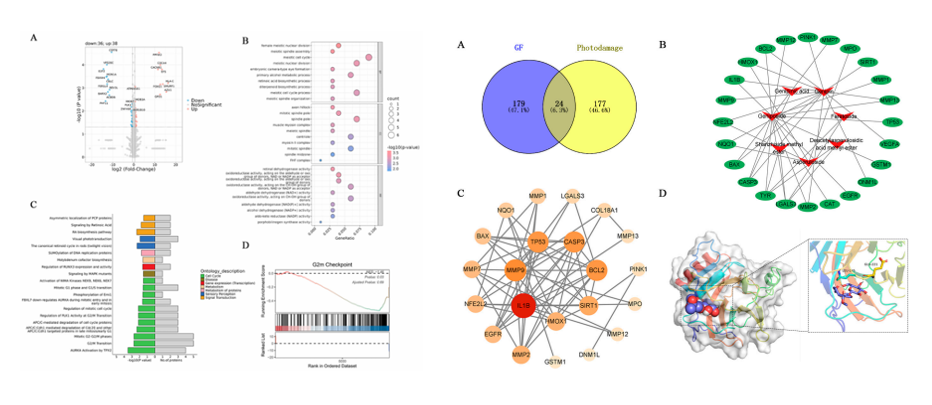
2. Application of Integrated Omics Technology
Combining proteomics and network pharmacology, DIA quantitative proteomics identified key anti-photodamage targets (e.g., G2/M checkpoint) in Gardenia extract. Network pharmacology analysis pinpointed core regulators (TP53, IL1B, MMP9). Geniposin-p53 molecular docking validation established a cross-omics verification paradigm for mechanistic studies of Gardenia extract's photoprotective effects.
CIRS Biotech has established an integrated in vitro efficacy evaluation mechanism encompassing whitening, anti-aging, sensitive skin, oil-control/acne removing, anti-glycation/antioxidation, and scalp care. By integrating biochemical experiment, cell models, 3D skin models, network pharmacology, skin microbiome models, and other cutting-edge technologies, CIRS Biotech has developed a multidimensional scientific assessment system that could meet the needs of cosmetic efficacy evaluation. This system:
- Quantitatively evaluates efficacy claims based on skin mechanisms;
- Elucidates active ingredients’ targets/pathways via bioinformatic network analysis;
- Predicts ingredient-protein interactions through molecular docking;
- Features a novel in vitro skin microbiome model to assess product effects on microbial quantity/diversity and ingredient safety under microbial metabolism.
Through interdisciplinary technology integration, CIRS Biotech delivers end-to-end solutions—from ingredient screening → efficacy verification → safety evaluation. Guided by a global scientific vision, CIRS Biotech advanced cosmetic efficacy assessment toward a data-centric and systematic paradigm, perpetually infusing scientific impetus into industry innovation and standardization upgrades.
Our Services
- In-vitro Efficacy Evaluation (Whitening, Moisturizing, Soothing, Anti-wrinkle/Firmness/Anti-aging, Oil control/Acne removal, Repairing, Anti-dandruff/Scalpcare, In-vitro Microbiome, Exfoliating)
- Invitro Safety Evaluation (OECD 471, OECD473, OECD487, OECD439, China SNIT 2329-2009, OECD492, OECD431)
If you need any assistance or have any questions, please get in touch with us via kexinbio@cirs-group.com.
